| |
What Molecules Has A Net Dipole Moment Of Zero

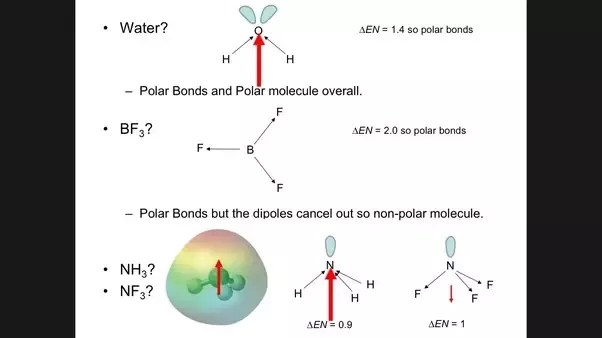
However since the molecule is linear these two bond dipoles cancel each other out ie.
What molecules has a net dipole moment of zero. So sigma would be on the hydrogen atom and sigma - would be on the Chlorine atom. Polar versus nonpolar molecues. The SO3 sulfur trioxide molecule has the highly symmetrical trigonal planar geometry and each one of the individual S-O bond in the species is equally polar.
The central carbon will have a net positive charge and the two outer oxygen atoms a net negative charge. PH 3 NH 3. The vector addition of the dipoles equals zero and the overall molecule has a zero net dipole moment.
However for b as both vectors are pointing in the same direction it has a net dipole charge. Using the cross bow arrow shown below we can show that it has a net dipole. It is the only non-polar molecule among the given five species and it has no net dipole moment.
While it may seem counterintuitive molecules may possess dipole moments yet entire molecules may still maintain no net dipole moment. BeH bonds are polar due to a difference in their electronegativity but the bond polarities cancel each other. Dipole moment is equal to the product of the partial.
NCERT P Bahadur IIT-JEE Previous Year Narendra Awasthi MS Chauhan. In contrast the H 2 O molecule is not linear part b in Figure 228. So the molecule has some net dipole moment and so it is polar.
Thus molecule has. Dipole moments can be thought of as vectors. All the other four molecules have net dipole moments and each one is a polar species.
Source : pinterest.com