| |
Which Species Has A Net Dipole Moment
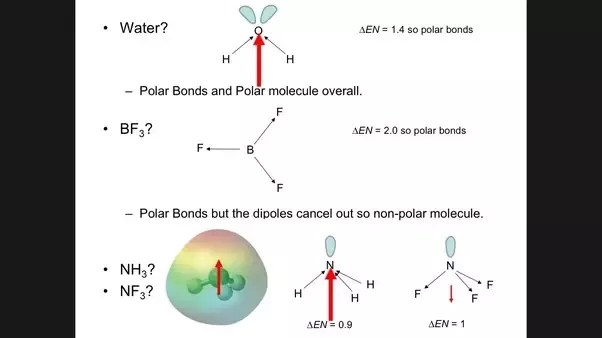
As a consequence X e F X 6 has net permanent dipole moment since all six X e X δ F X δ bonds are polar.
Which species has a net dipole moment. Select all that apply. This occurs due to an atoms electronegativity - where one atom has the ability to attract electrons towards it In other words electrons wants to spend other time around it giving it a negative charge and the other a positive charge. A Each CO bond has a bond dipole moment but they point in opposite directions so that the net CO 2 molecule is nonpolar.
What is the difference between a permanent and a temporary dipole. Select all that apply. A dipole is formed by the pair of equally and oppositely charged species.
Polar covalent bonds behave as if the bonded atoms have localized fractional charges that are equal but opposite ie the two bonded atoms generate a dipole. As a result the CO 2 molecule has no net dipole moment even though it has a substantial separation of charge. This charge polarization allows H 2 O to hydrogen-bond to other polarized or charged species.
Which species have no dipole moment. The three fluorine atoms are so attached to the boron that the resultant of dipole moment of two fluorine atoms cancel with the third one 176K views. For your question about ammonia look at Ivan Neretins comment and here where there is an accepted answer.
Greater the electronegative difference more is the dipole moment. Dipole moment arises by the difference in the electronegativities of the atoms in the molecule. If the structure of a molecule is such that the individual bond dipoles do not cancel one another then the molecule has a.
Because the two CO bond dipoles in CO 2 are equal in magnitude and oriented at 180 to each other they cancel. The polarity of a molecule is also depending on the dipole moment. Each CO bond in CO 2 is polar yet experiments show that the CO 2 molecule has no dipole moment.
Source : pinterest.com